
Where X is the energy level corresponding to the principal quantum number n, type is a lower-case letter denoting the shape or subshell of the orbital and it corresponds to the angular quantum number l, and y is the number of electrons in that orbital.įor example, the orbital 1 s 2 ( pronounced "one ess two") has two electrons and is the lowest energy level ( n = 1) and has an angular quantum number of l = 0. 8 Electron placement and the periodic table.For mnemonic reasons, some call them spherical & peripheral. The orbital names (s, p, d, f, g, h.) are derived from the characteristics of their spectroscopic lines: sharp, principal, diffuse and fundamental, the rest being named in alphabetical order. Hence the term "orbit" was substituted with something else: orbital.
As electrons cannot be described as solid particles (as a planet or a moth) in this way, a more accurate analogy would be that of a huge atmosphere, the spatially distributed electron, around a tiny planet which is the atomic nucleus. In quantum mechanics, atomic orbitals are described as wave functions over space, indexed by the n, l, and m quantum numbers of the orbital or by the names as used in electron configurations, as shown on the right. Explaining the behavior of the electrons that "orbit" an atom was one of the driving forces behind the development of quantum mechanics. Specifically, atomic orbitals are the possible quantum states of an individual electron in the electron cloud around a single atom, as described by the function.Ĭlassically, the electrons were thought to orbit the atomic nucleus, much like the planets around the Sun (or more accurately, a moth orbiting very quickly around a lamp). The term "orbital" has become known as either the "mathematical function" or the "region" generated with the function. The region in which an electron may be found around a single atom in a particular energy state can be calculated from this function. All the four hydrogen atoms are arranged in a manner such that the four hydrogen atoms form corners of a regular tetrahedron.An atomic orbital is a mathematical function that describes the wave-like behavior of an electron in an atom.

According to experimental observations, the Methane molecule has 4 identical C-H bonds with equal length and equal bond energy. The above are three basic hybridizations along with them there are other hybridizations based on the mixing of orbitals such as sp³d hybridization, sp³d² hybridization and sp³d² hybridization.Įxplanation of Hybridization Through ExamplesĮxample 1: Consider an example of the simplest hydrocarbon molecular Methane. The molecules possessing sp hybridization used to have a linear shape with an angle of 180°.
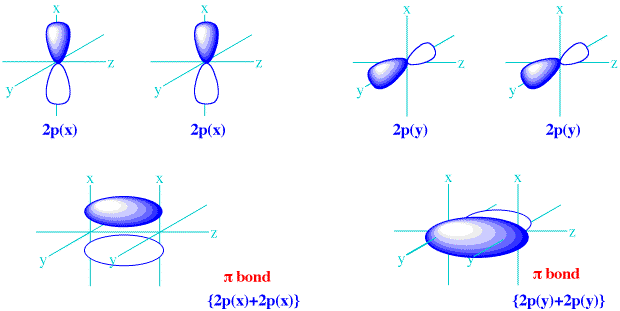
Sp hybridization: When one s and one p orbital goes in the process of mixing of energy to form a new orbital such kind of hybridization is called sp hybridization. Sp² hybridization: It is observed when one s orbital and two p orbitals undergo mixing of energy for equivalent orbitals.

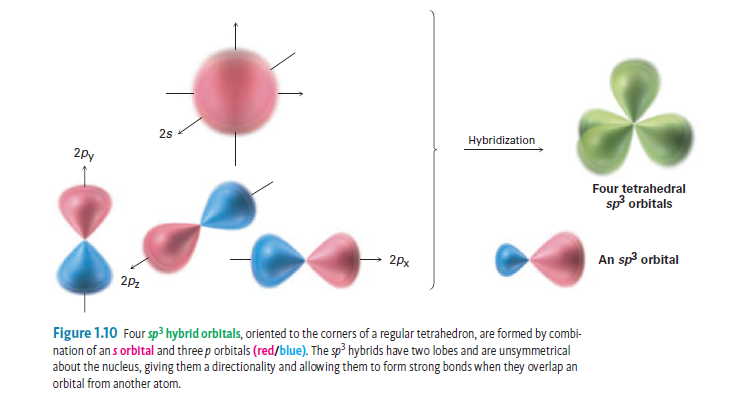
Sp³ hybridization: When one s orbital and three p orbital from the same shell of atom mix together to form a new equivalent orbital then this is called sp³ hybridization. There are different types of hybridization-based on the mixing of the orbitals. This theory is especially useful to explain the covalent bonds in organic molecules.īasically, hybridization is intermixing of atomic orbitals of different shapes and nearly the same energy to give the same number of hybrid orbitals of the same shape, equal energy and orientation such that there is minimum repulsion between these hybridized orbitals. Hybridization is a concept used in organic chemistry to explain chemical bonding in cases where the valence bond theory does not provide satisfactory clarification.


 0 kommentar(er)
0 kommentar(er)
